The entire program is based on the integration of a transdisciplinary approach from the basic sciences in the field of immunological, virological and translational research into the development of a clinical therapeutic and prophylactic program.
The program is developed at the national level with the VRI teams in France but also internationally with, among others, the Baylor Institute for Immunology Research (BIIR, USA, Dr Gerard Zurawski), historical partner of the VRI, with Duke University (USA, Prof Barton Haynes), with CHUV (Switzerland, Prof Giuseppe Pantaleo) and through the coordination by the VRI of the European consortium H2020 EHVA (European HIV Vaccine Alliance) on HIV vaccines.
Thanks to the VRI structuration of the field since 2011, the VRI program concentrates along three axes:
- To understand the mechanisms of action of candidate vaccines and immunomodulators
- To develop the new generation DC-targeting vaccines
- To pursue a clinical development plan focused on DC-based vaccine strategies
These three areas of research are carried out by the 7 divisions of the VRI – basic research, DC-based vaccines, preclinical models, clinical development, immunological monitoring, virological evaluation, and data science. The divisions evolve to align with specific scientific program needs and technological and scientific innovations of the sector.
An integrated approach
VRI’s scientific strategic plan is organized in 6 integrated research programs and structured around common scientific, logistic and administrative cores to conduct researches aimed at developing an effective vaccine against HIV/AIDS, HCV and emerging infectious diseases.
The main objective is the production of vaccine candidates targeting dendritic cells (DC), which play a key role in the induction and regulation of specific immunity. The targeting of dendritic cells is carried out using monoclonal antibodies directed against receptors expressed on the surface of DCs, and coupled to an antigen. This type of vaccine can induce humoral responses (antibodies) and / or T cell responses, and thus be used for preventive or therapeutic settings vaccination.
DC vaccines, viral vectors
Presentation of the antigen
Impact of vaccines on antigen presenting cells
Preclinical models
In vivo selection of vaccines
Clinical trials
In vivo immunogenicity
Protective immune correlates
Vaccinology system
Mucosal and cellular responses B
The whole program is based on the integration of a translational research approach from basic science towards clinic
Major achivements
The development of new vaccin
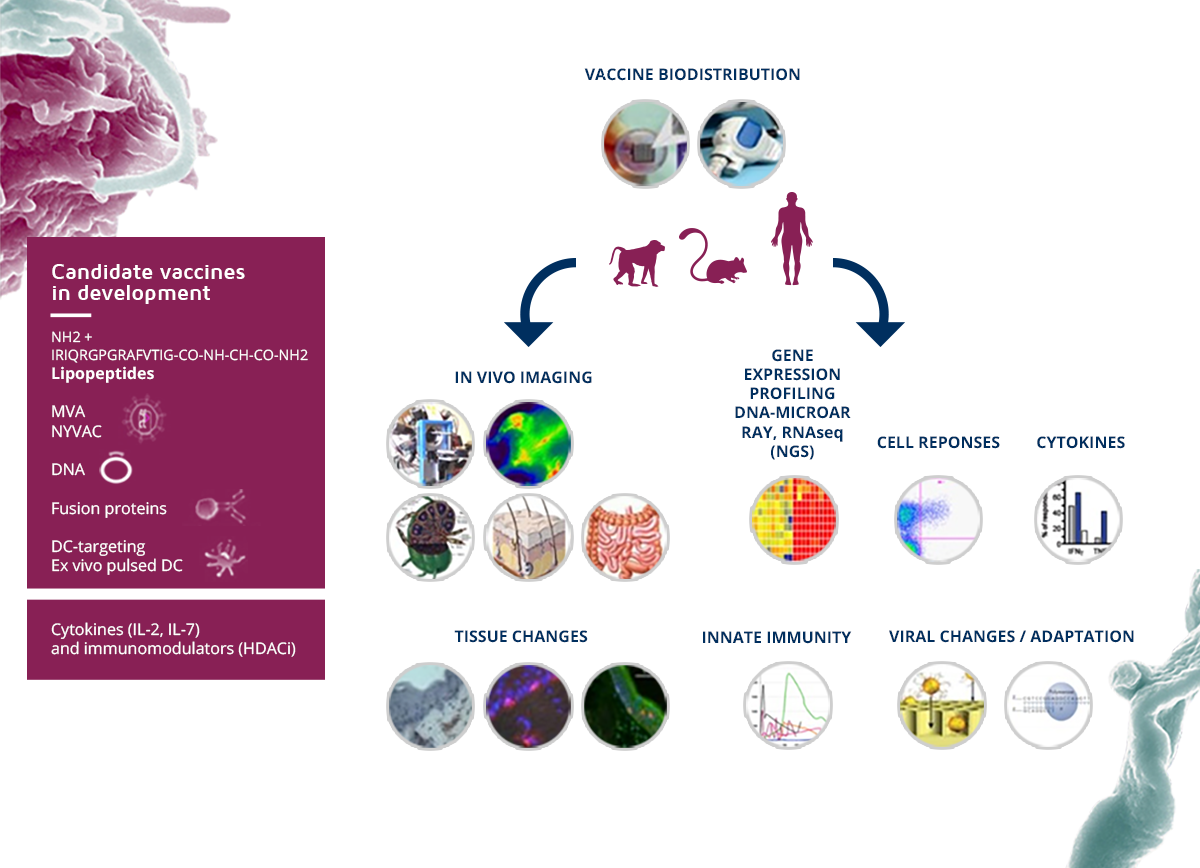
In order to develop and extend the range of available vaccine candidates for HIV and (re)-emerging infectious diseases, based on various technologies such as the use of lipopeptides, DNA vaccines, vaccines using Recombinant viral vectors, the VRI has developed innovative vaccines targeting dendritic cells (DC), with two types of vaccine candidates: (i) the construction of fusion proteins associating monoclonal antibodies targeting dendritic cells and coupled with HIV proteins (T epitopes and envelope proteins), (ii) as well as the generation of ex-vivo DCs loaded with HIV lipopeptide epitopes (development in phase I / II).
Preclinical evaluation and selection of the best vaccine candidates targeting DC. production in accordance with good manufacturing practice (cGMP) and the release of the first clinical batches of this HIV DC-targeting vaccine in 2019 constitutes a major progress in the VRI's vaccine program. A clinical trial with this new vaccine will be carried out in 2020.
The development of innovative platforms
To better characterize the in vivo responses of our portfolio of vaccine candidates, preclinical animal models , cutting-edge equipment and technologies for immuno and viromonitorinng integrated analyzes of multiparametric mass spectrometry or CyTOF, next generation in vivo imaging and sequencing have been implemented at the VRI . The integrated analysis of large-scale data generated during preclinical studies and systematically performed clinical trials is performed in a system vaccinology strategy and aims to identify the correlates of protection or the control of viral replication and allow the development of new models of vaccine strategies. Our long-term goal, in addition to developing vaccines in phase IIb clinical trials, is to model the mechanisms of the vaccine response to optimize resources and accelerate the clinical development of vaccine candidates. These tools will be decisive for the evaluation of other vaccines in (re)-emerging infectious outbreaks or experimental treatments for therapeutic purposes.
Clinical Trial
The VRI is developing two types of clinical trials for vaccines against HIV and (re)-emerging infectious diseases : clinical trials aimed at developing a preventive vaccine, involving healthy volunteers, and trials of therapeutic vaccines, conducted for example in the case of HIV on people living with HIV.