Monkeypox: characterization of post-infectious immune response
JUNE 06, 2023
Press release
In 2022-2023, an outbreak of monkeypox, now known as mpox (caused by the monkeypox virus or MPXV) led to 87,000 human cases in 170 countries1. Most cases were reported outside the usual areas in which the virus circulates. Since the outbreak began, surveillance of the virus has been stepped up in Europe, with nearly 5,000 cases being reported in France2. Scientists and clinicians from the Institut Pasteur, the CNRS, Inserm, the VRI and the Paris Public Hospital Network (AP-HP) studied 470 sera from vaccinated or MPXV-infected individuals to elucidate the mechanisms involved and determine correlates of protection against infection or disease severity3. They determined the sensitivity of the virus to neutralizing antibodies and analyzed the immune response of these vaccinated or MPXV-infected individuals. The study revealed the role of complement4, a component of the innate immune system, in this response. The findings were published in the journal Cell Host & Microbe on May 4, 2023.
In 2022-2023, an unprecedented epidemic of 87,000 cases of mpox occurred in non-endemic areas, affecting people with no direct link to travel in Central or West Africa, where the virus has historically been present. MPXV is mainly transmitted to humans by rodents, with human-to-human transmission occurring via respiratory droplets or close contact. Symptoms are less severe than those of smallpox, and the case-fatality rate is lower. According to Santé publique France, approximately 5,000 cases of MPXV infection have been reported in France since May 2022.2 MPXV is still circulating at very low levels in non-endemic areas, which is why it is important to improve characterization and analyze the immune response of people infected with the virus or vaccinated with IMVANEX, the third-generation vaccine currently available, initially developed for smallpox.
The research teams worked in collaboration with clinicians, vaccinologists and virologists from three French hospitals (Henri Mondor Hospital in Créteil, La Pitié-Salpêtrière Hospital and Orléans Hospital) to carry out this multidisciplinary research. The large number of sera analyzed provided good statistical power, meaning that the analysis could be narrowed to subgroups of patients based on various criteria such as age.
In this study, published in Cell Host & Microbe, the leading journal on interactions between microbes and the immune system, the scientists studied the sensitivity of MPXV to neutralizing antibodies (NAbs) generated after infection with the virus and/or vaccination with IMVANEX. The IMVANEX vaccine has been used as pre- and post-exposure prophylaxis in high-risk populations, but its effectiveness is not yet well characterized. To analyze the sensitivity of the virus, the team of scientists developed two cellular tests to quantify neutralizing antibodies, using either the attenuated virus as a vaccine (MVA) or an MPXV strain isolated in a recently infected individual.
The study demonstrated the role of complement,4 already known for other poxviruses, and the neutralizing activity of the antibodies generated by infection or vaccination. Robust levels of anti-MVA antibodies were detected after infection, vaccination with the historic smallpox vaccine, or administration of IMVANEX or another MVA-based vaccine candidate. MPXV was minimally sensitive to neutralization in the absence of complement. The addition of complement from sera enhanced detection of individuals with antibodies and increased their level of anti-MPXV antibodies. Four weeks after infection, anti-MVA and -MPXV NAbs were observed in 94% and 82% of individuals, respectively. Two doses of IMVANEX generated anti-MVA and -MPXV NAbs that were detectable in 92% and 56% of vaccinees, respectively.
The highest level of antibodies was found in individuals born before 1980 (who had therefore been vaccinated for smallpox), whether after infection or after administration of IMVANEX, highlighting the impact of historic smallpox vaccination on immune responses to infection or administration of IMVANEX. This suggests that a sort of hybrid immunity was generated in infected individuals who were vaccinated in childhood.
The number of MPXV infections has been constantly on the rise since mass vaccination for smallpox was discontinued in the 1980s. “The neutralization assays developed in connection with this research may help define correlates of protection against infection or disease severity. The assays can also be used to conduct epidemiological surveys, assess the duration of protection conferred by previous infection or by authorized and candidate vaccines, and analyze the use of immunotherapeutic intervention. The assays represent useful tools to understand the mechanisms of multiplication of MPXV and its effects on public health, and to optimize patient treatment,” commented Olivier Schwartz, Head of the Institut Pasteur’s Virus and Immunity Unit and last author of the study.
To find out more about mpox, see the fact sheet at pasteur.fr
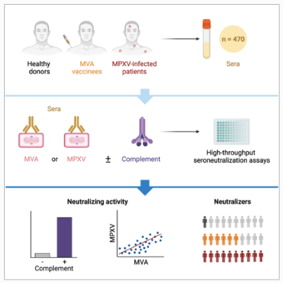
Summary of the main points of the study
Development of two poxvirus seroneutralization assays using MVA or MPXV.
Complement enhances neutralizing activity of antibodies (NAbs).
Historic smallpox vaccination increases the level of antibodies induced by infection or MVA vaccination.
Source
Complement-dependent mpox virus-neutralizing antibodies in infected and vaccinated individuals, Cell Host & Microbe, 4 mai 2023
Mathieu Hubert1, Florence Guivel-Benhassine1, Timothée Bruel1, Françoise Porrot1, Delphine Planas1, Jessica Vanhomwegen2, Aurélie Wiedemann3, Sonia Burrel4, Stéphane Marot5, Romain Palich6, Gentiane Monsel6, Harouna Diombera3, Sébastien Gallien7, Jose Luis Lopez-Zaragoza7, William Vindrios7, Fabien Taieb8, Sandrine Fernandes-Pellerin9, Maurine Delhaye9, Hélène Laude9, Laurence Arowas9, Marie-Noelle Ungeheuer9, Laurent Hocqueloux10, Valérie Pourcher6, Thierry Prazuck10, Anne-Geneviève Marcelin5, Jean-Daniel Lelièvre3,7, Christophe Batéjat2, Yves Lévy3,7, Jean-Claude Manuguerra2, Olivier Schwartz1,11,*
1 Institut Pasteur, Université Paris Cité, Virus and Immunity Unit, CNRS UMR3569, 75015 Paris, France, & Vaccine Research Institute, 94000 Créteil, France.
2 Institut Pasteur, Université Paris Cité, Unité Environnement et Risques Infectieux, Cellule d’Intervention Biologique d’Urgence (CIBU), 75015 Paris, France
3 Vaccine Research Institute, Université Paris-Est Créteil, Faculté de Médecine, INSERM U955, Team 16, 94000 Créteil, France
4 Université de Bordeaux, CNRS UMR 5234, Fundamental Microbiology and Pathogenicity, Hôpital Universitaire de Bordeaux, Service de Virologie, 33000 Bordeaux, France
5 Sorbonne Université, INSERM, Institut Pierre Louis d’Epidémiologie et de Santé Publique, AP-HP, Hôpitaux Universitaires Pitié-Salpêtrière – Charles Foix, Laboratoire de Virologie, 75013 Paris, France
6 Sorbonne Université, INSERM 1136, Institut Pierre Louis d’Epidémiologie et de Santé Publique, Assistance Publique – Hôpitaux de Paris, Hôpitaux Universitaires Pitié-Salpêtrière Charles Foix, Service de Maladies infectieuses et Tropicales, 75013 Paris, France
7Assistance Publique-Hôpitaux de Paris, Groupe Henri-Mondor Albert-Chenevier, Service Immunologie Clinique, 94000 Créteil, France
8 Medical Center of Institut Pasteur, 75015 Paris, France
9 ICAReB-Clin platform, Institut Pasteur, 75015 Paris, France
10 CHR Orléans, Service de Maladies Infectieuses, 45100 Orléans, France
11 Lead contact
* Corresponding author
Contact
Service de presse de l’Institut Pasteur
MYRIAM REBEYROTTE 01 45 68 81 01
ANNE BURLET-PARENDEL 01 86 46 79 32
AURELIE PERTHUISON 01 45 68 89 28
presse@pasteur.fr
Notes
-
1. WHO figures.
-
2. Santé publique France. Mpox (MPXV): the latest situation in France at April 27, 2023.
-
3. This study was supported by ANRS | Emerging Infectious Diseases, which provided samples for research.
-
4. Complement is a system of proteins in serum that contributes to the body’s defense. It is involved in mechanisms to eliminate pathogens. Institut Pasteur scientist Jules Bordet was awarded the Nobel Prize in Physiology or Medicine in 1919 for his research on the role of the complement system and antibodies.