New discovery concerning receptors used by coronaviruses to enter human cells
OCTOBER 25, 2023
Press release
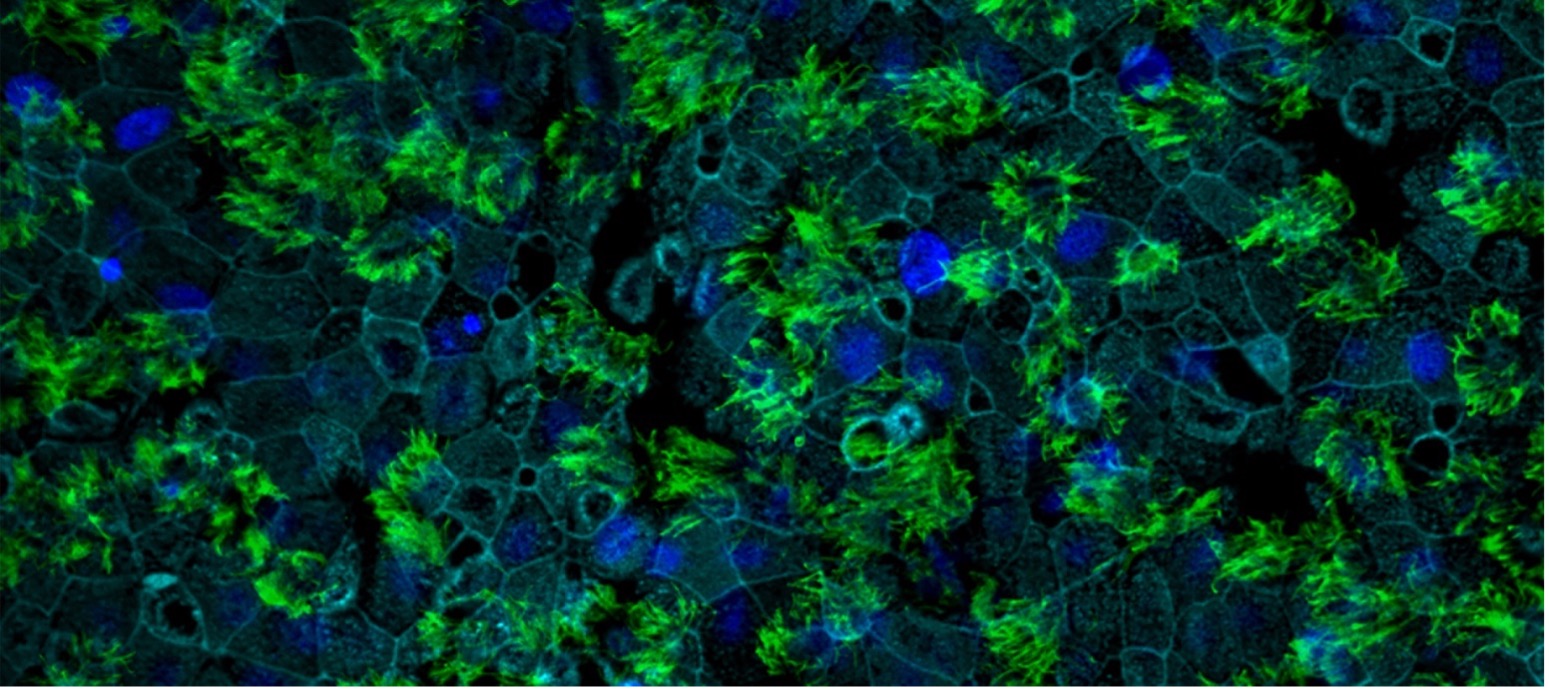
Human respiratory tissue with ciliated cells stained green (anti-TMPRSS2 antibodies), nuclei in dark blue and cell membranes in light blue. © Vincent Michel, Institut Pasteur
The SARS-CoV-2 virus responsible for COVID-19 can cause severe acute respiratory syndrome, contrasting with other coronaviruses that were known to cause mild seasonal colds prior to its emergence in 2019. This raises the question of why one coronavirus affects humans more severely than another. Scientists at the Institut Pasteur, Université Paris Cité and the VRI have now provided part of the answer by identifying a gateway used by the seasonal coronavirus HKU1 to enter human cells. HKU1 binds to a different receptor than SARS-CoV-2, which may partly explain the difference in severity between these two coronaviruses. Receptors provide a useful means of elucidating coronavirus transmissibility and pathology as part of surveillance work on viral evolution. These results are published in the October 25, 2023 issue of Nature.
Seven coronaviruses are known for their ability to infect humans. Four of these are generally mild: HKU1, 229E, NL63 and OC43, while the other three are more pathogenic: SARS-CoV-1, Mers-CoV and SARS-CoV-2.
The HKU1 virus was first identified in an elderly patient with severe pneumonia in Hong Kong in 2005. Like SARS-CoV-2, HKU1 mainly infects upper respiratory tract cells. However, it rarely affects the bronchi and alveoli in the lungs. The HKU1 virus causes colds and other mild respiratory symptoms. Complications may also occur, including severe respiratory tract infections, particularly in young children, the elderly and immunocompromised individuals. It is estimated that 70% of children are infected before the age of 6. In total, 75 to 95% of the global population has been exposed to HKU1, which is comparable to other seasonal human coronaviruses.
At cellular level, coronavirus spike proteins are cleaved, or split in two, after binding to their receptors. This cleavage phenomenon is vital for viral fusion, entry and multiplication. Some coronaviruses (SARS-CoV-2 and NL63) use the ACE2 receptor as a gateway for entering cells. Until now, HKU1 and OC43 were the only coronaviruses with unknown receptors.
Through collaboration between scientists at eight Institut Pasteur units, it was possible to identify the TMPRSS2 enzyme as the receptor to which HKU1 binds to enter cells. Once binding has occurred, TMPRSS2 triggers fusion of HKU1 with the cell, leading to viral infection. Through a combination of techniques performed in vitro and in cell culture, the scientists demonstrated that the TMPRSS2 receptor has high affinity with the HKU1 spike, which is not the case for SARS-CoV-2.
“Once a receptor has been identified for a virus, it is possible to characterize target cells more accurately, while also gaining insights on viral entry and multiplication mechanisms and infection pathophysiology,” comments, Olivier Schwartz, co-last author of the study and Head of the Institut Pasteur’s Virus and Immunity unit.
“Our findings also shed light on the various evolution strategies employed by coronaviruses, which use TMPRSS2 either to bind to target cells or trigger fusion and viral entry,” adds Julian Buchrieser, co-last author of the study and scientist in the Institut Pasteur’s Virus and Immunity unit.
These human-pathogenic viruses’ use of different receptors probably affects their degree of severity. Receptor levels vary among respiratory tract cells, thus influencing the sensitivity of cells to infection and viral spread. Once the route of viral entry into cells is known, it should also be possible to fight infection more effectively by developing targeted therapies and assess the risk of virulence posed by any future emerging coronaviruses.
In parallel with this work, Institut Pasteur teams led by Pierre Lafaye and Felix Rey have developed and characterized nano-antibodies (very small antibodies) that inhibit HKU1 infection by binding to the TMPRSS2 receptor. These reagents have been patented for potential therapeutic activities.
This work was funded by the above-mentioned research bodies with additional support from the French Foundation for Medical Research (FRM), ANRS-Emerging Infectious Diseases, Vaccine Research Institute, the European HERA DURABLE project, the Labex IBEID and the ANR/FRM Flash Covid project.
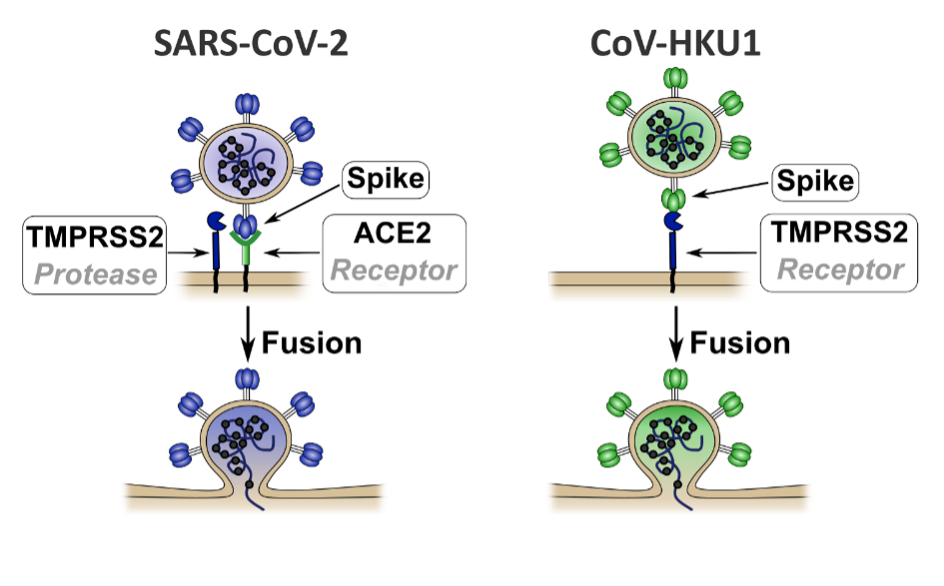
Different routes of entry into human cells for SARS-CoV-2 and HKU1. SARS-CoV-2 binds to ACE2 protein. The protease TMPRSS2 then cleaves the spike to enable viral fusion and entry into the target cell. HKU1 binds directly to TMPRSS2 in order to enter the cell without using ACE2. © Institut Pasteur
Sources
TMPRSS2 is a functional receptor for human coronavirus HKU1, Nature, 25 octobre 2023
Nell Saunders1, Ignacio Fernandez2, Cyril Planchais3, Vincent Michel4, Maaran Michael Rajah1, Eduard Baquero Salazar5, Jeanne Postal1, Francoise Porrot1, Florence Guivel-Benhassine1, Catherine Blanc6, Gaëlle Chauveau-Le Friec7, Augustin Martin1, Ludivine Grzelak1, Rischa Maya Oktavia2, Annalisa Meola2, Olivia Ahouzi2, Hunter Hoover-Watson1, Matthieu Prot8, Deborah Delaune8,13, Marion Cornelissen9,10, Martin Deijs10,11, Veronique Meriaux7, Hugo Mouquet3, Etienne Simon-Lorière8,14, Lia van der Hoek10,11, Pierre Lafaye7, Felix Rey2, Julian Buchrieser1,#,* and Olivier Schwartz1,12,#,*
1Virus & Immunity Unit, Institut Pasteur, Université de Paris Cité, CNRS UMR 3569, Paris, France
2Structural Virology Unit, Institut Pasteur, Université de Paris Cité, CNRS UMR 3569, Paris, France
3Humoral Immunology Unit, Institut Pasteur, Université de Paris Cité, INSERM U1222, Paris, France
4Pathogenesis of Vascular Infections Unit, Institut Pasteur, INSERM, Paris, France.
5Nanoimaging core, Institut Pasteur, Université de Paris Cité, INSERM U1222, Paris, France
6Pasteur-TheraVectys Joint Lab, Institut Pasteur, Université de Paris Cité, Paris, France
7Antibody Engineering Platform, C2RT, Institut Pasteur, Université de Paris Cité, CNRS UMR 3528, Paris, France.
8G5 Evolutionary Genomics of RNA Viruses, Institut Pasteur, Paris, France.
9Department of Medical Microbiology and Infection Prevention, Amsterdam UMC, Molecular Diagnostic Unit, University of Amsterdam, 1105 AZ Amsterdam, Netherlands.
10Amsterdam Institute for Infection and Immunity, Amsterdam, The Netherlands
11Department of Medical Microbiology and Infection Prevention, Amsterdam UMC, Laboratory of Experimental Virology, University of Amsterdam, 1105 AZ Amsterdam, Netherlands.
12Vaccine Research Institute, Creteil, France
13Institut de Recherche Biomédicale des Armées, Brétigny-sur-Orge, France
14National Reference Center for viruses of respiratory infections, Institut Pasteur, Paris, France.
#Co-last authors
*Corresponding authors
Contact
Institut Pasteur Press Office
MYRIAM REBEYROTTE 01 45 68 81 01
ANNE BURLET-PARENDEL 01 86 46 79 32
AURELIE PERTHUISON 01 45 68 89 28
presse@pasteur.fr